Explore insights by category
Articles | Case studies and testimonials | E-books | Guides | On-demand webinars | White papers | Videos | View all
Latest featured insights
Video
Optum Life Sciences leaders break down common missteps when using RWD and how to create practical strategies to overcome them. Watch the video from STAT Summit.
E-book
Life sciences market experts from Optum and Advisory Board share perspectives on trends that will influence the future drug value chain.
Article
Cut through the noise of the Inflation Reduction Act of 2022 with key points aimed to help life sciences leaders leverage impactful data.
Guide
Expanding the use of RWD requires expertise and leadership. Learn how to maximize the success of a centralized RWD team.
Latest articles
Article
Cut through the noise of the Inflation Reduction Act of 2022 with key points aimed to help life sciences leaders leverage impactful data.
Article
To maximize the value of clinical innovations, you need dimensional, robust data. Learn about leveraging integrated cost and clinical data.
Latest case studies and testimonials
Case study
Optum Life Sciences experts used real-world data to assist in the testing of an algorithm designed to assess for osteoporosis risk.
Case study
States can build on a step-by-step approach to modernization, incrementally transforming the user experience without disruption.
Case study
Using our Voice-to-Claim® methodology to better understand the attitudes and behaviors of patients and physicians and their effects on cost and utilization.
Latest e-books
E-book
Life sciences market experts from Optum and Advisory Board share perspectives on trends that will influence the future drug value chain.
E-book
Explore real-world use cases to see how integrated data empowers research and commercial teams to compete in today’s crowded market.
Latest guides
Guide
Expanding the use of RWD requires expertise and leadership. Learn how to maximize the success of a centralized RWD team.
Guide
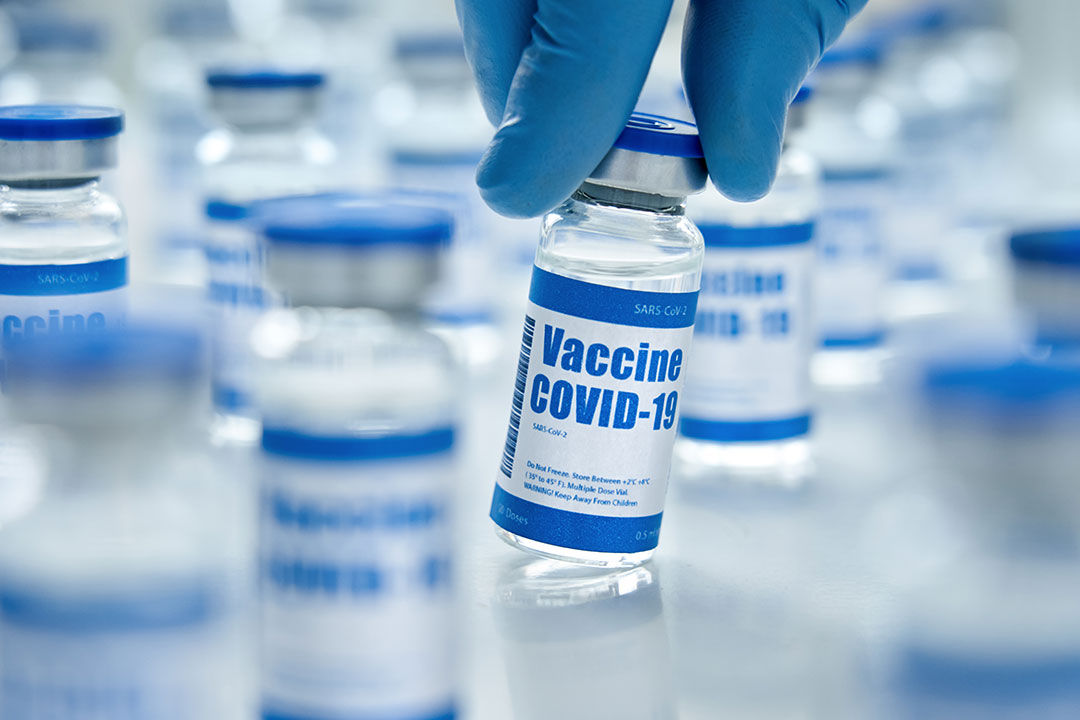
As a key FDA collaborator, Optum Epidemiology generates reproducible results that inform regulatory decisions for COVID-19 vaccines.
Latest on-demand webinars
On-demand webinar
To meet the requirements, states should consider focusing on five key areas.
On-demand webinar
Optum experts give a snapshot of commonly encountered pitfalls when using RWD and recommendations to help avoid them in this PMSA webinar.
Latest white papers
Guide
Expanding the use of RWD requires expertise and leadership. Learn how to maximize the success of a centralized RWD team.
White paper
Read how you can fill the data divide to develop effective treatment options.
White paper
Real-world data can help evaluate the effectiveness of medications and therapies, which is why evaluation should be part of the investment.
Latest videos
Video
Optum Life Sciences leaders break down common missteps when using RWD and how to create practical strategies to overcome them. Watch the video from STAT Summit.
Video
We design programs between life sciences companies, payers, providers and patients to improve care and patient and provider satisfaction.